Abstract
Introduction
Patterned hair loss (PHL) continues to be one of the most important hair problems affecting both men and women. Long before the clinical characterization of this entity, occurrence of age-dependent form of scalp hair loss was well known in women. Female pattern hair loss (FPHL) is characterized by a reduction in hair density over the crown and frontal scalp with retention of the frontal hairline. The term androgenetic alopecia (AGA), more commonly used for patterned baldness in men (male AGA or MAGA), is often used synonymously with FPHL. Even Ludwig, credited with classification of FPHL, used the term AGA for this entity despite acknowledging its clinical and patho-physiologic differences from MAGA. [1] Owing to the uncertain relationship between androgens and this entity, and increasing identification of androgen-independent mechanisms in its pathophysiology, the FPHL has emerged as the preferred term for this condition. However, interchangeable use of these terms continues to prevail. The different facets of FPHL, such as epidemiology, clinical features, differential diagnoses, pathophysiology, adolescent AGA, evaluation, treatment, and association with metabolic syndrome have been succinctly reviewed in this article.
Methods
Literature since inception to 2012 was reviewed via MEDLINE/PUBMED using the key words alopecia, androgenetic, women, hair loss, female, prevalence, adolescent, classification, treatment, metabolic syndrome, and cardiovascular. As there is a dearth of high-quality trials in FPHL, it was deemed worthwhile to include all level researches.
Epidemiology
In view of the global importance of FPHL, it is worthwhile to recount its epidemiological aspect. FPHL is more frequent than previously thought; the incidence of PHL in women may equal men. [2] FPHL may begin at any age following puberty and it is widely acclaimed that the prevalence increases post-menopause with a possible hormonal influence. [3] In a community-based study from China, the prevalence of FPHL was 6.0% across all age groups ranging from 1.3% in the age group of 18-29 years, increasing steadily with age to 10.3% in the seventh decade and 11.8% thereafter; with a positive family history in 19.2%. [4] In Korean women, the prevalence of AGA at all ages was 5.6%, 0.2% in the third, 3.8% in the fifth, and 7.4% in the sixth decade. It was 11.7% and 24.7%, respectively, in the seventh decade and thereafter. Grade III was not observed and a positive family history was present in 45.2%. [5] In UK, 6% women aged less than 50 years were diagnosed as having FPHL, increasing to 38% in subjects aged 70 years and above. [6] In Maryborough (Australia), 64.4% of women above 20 years of age exhibited bitemporal hair loss. Overall, the prevalence of mid-frontal hair loss increased with age and affected 57% of women aged 80 years and above. [7] In another study comprising 1006 Caucasian women, the FPHL was found to be common beginning in the late 20s and reaching its peak after 50 years of age. [8] No concrete data are available from the Indian subcontinent.
Pathophysiology
FPHL is a multi-factorial entity. Like MAGA, the FPHL tends to occur in genetically pre-disposed patients with altered hair follicle cycling and miniaturization of hair follicles leading to the transformation of terminal to shorter and finer vellus hair follicles. Histologically, it is thought to be caused by a reduction in dermal papilla volume. [9] The duration of anagen shortens dramatically from 3 to 6 years to few weeks or months, whereas the duration of telogen remains the same or lengthens to more than 3 months resulting in an accelerated turnover of anagen hair and significant increase in the proportion of telogen hair from 5-10% to approximately 15-20%. [10] The latter is responsible for the increased hair shedding following combing and washing.
Role of androgens in female pattern hair loss
The androgen receptor (AR) and three important enzymes, 5α-reductase I, II, and aromatase, are present in the outer root sheath and dermal papillae of hair follicles of both men and women with AGA. The AR content in female frontal hair follicles is 40% lower than that in the male follicles. Frontal hair follicles in women have 3 and 3.5 times less 5α-reductase I and II levels, respectively. However, aromatase content in frontal hair is six times higher in women. [11] This may, in part, explain the difference in clinical expression of PHL between men and women.
It is imperative at this point to recapitulate the mechanism of action of androgens in hair follicles [Figure – 1]. Conversion of testosterone (T) to dihydrotestosterone (DHT) requires free T that is not bound to sex-hormone-binding globulin (SHBG).
 |
Women with FPHL are more likely to show signs of virilization and some reveal hyperandrogenemia. In a study comprising 109 women with FPHL, 38.5% were found to have a clinical or biochemical evidence of hyperandrogenism. [12] Of the 187 women with hair loss, 67% of those with hair loss alone and 84% who were also hirsute were found to have some degree of biochemical androgen access. [13] In yet another study comprising 89 women with hair loss, 67% showed ultrasound evidence of polycystic ovaries in contrast to 27% in control group (n = 73). Besides, 21% of the former were significantly hirsute as compared with 4% in the latter. [14] On the contrary, other investigators have failed to find the evidence of raised androgens in FPHL. [15],[16] Androgens may also act through yet unknown non-AR mechanisms as emphasized in an interesting report of a woman with complete androgen insensitivity syndrome manifesting FPHL. [17]
Role of estrogens in female pattern hair loss
The role of estrogens for the normal development of pubic and axillary hair in females is well known but its role in scalp hair growth in both sexes is less clear and less studied. Enzymes aromatase and 17β-hydroxysteroid dehydrogenase expressed within dermal papilla cells and keratinocytes of the outer root sheath of hair follicle, metabolize estrogens. Aromatase is the key enzyme involved in the conversion of androgens to estrogens in tissue sites (e.g., T into 17β-estradiol and androstenedione into estrone). [18] Estrogens have been shown to modify androgen metabolism within dermal papilla cells by reducing the DHT production from T either as a result of direct inhibition of 5α-reductase or through estrogen-induced conversion of T into weaker steroids. [19]
There is controversy as to whether estrogens are stimulatory or inhibitory to hair growth. Its stimulatory role is evident by the increased prevalence of FPHL after menopause. There is prolongation of anagen phase during pregnancy secondary to high systemic estrogen whereas post-partum fall in estrogen levels may partially account for the simultaneous conversion of hair follicles into the telogen phase, resulting in the condition telogen gravidarum. [19] Aromatase inhibitor therapies leading to lower estrogen levels have been observed to induce hair loss, and topical estrogen applications have been widely used as hair growth stimulants in FPHL. Conversely, estrogens have been shown to inhibit hair growth in murine animal models. An aromatase gene variant associated with higher circulating estrogen levels is known to occur at higher frequencies in women with FPHL compared with unaffected women. [20]
Recently, the role of autoimmunity in PHL has been speculated. A lymphocytic microfolliculitis around the bulge epithelium along with deposits of epithelial basement membrane zone immunoreactants are frequent findings in AGA. Indeed, those showing a positive immunoreactant profile respond well to combination therapy with minocycline and topical steroids along with red light compared with those with a negative result. [21] In addition, levels of caspase-1 (an expression of innate immunity) have been found to be greater in men with AGA. In vivo and in vitro finasteride treatment results in lower caspase-1 expression and, therefore, androgens may influence innate immunity involved in the hair cycle in AGA. [22] This needs to be looked into in FPHL also.
Genetic pre-disposition is a pre-requisite and a polygenic mode of inheritance for FPHL has now been well established.These genes may determine the age of onset, progression, pattern, and severity. [23] Attempts have been made to identify genetic alteration in AGA. The AR/ectodysplasin A2 receptor locus on the X-chromosome has been identified to pre-dispose to FPHL. [24] A study from the USA concluded that AR-CAG repeats, in conjunction with epigenetic factors, may determine which women with hair loss will respond to finasteride therapy. [25] However, a report from Japan found that the efficacy of finasteride in each FPHL patient cannot be predicted by the CAG repeat numbers in the AR gene. [26] In addition, sequence variation in the gene encoding aromatase, CYP19A1, may influence the risk of developing FPHL. [27] A recent meta-analysis revealed a significant association between the G allele of the AR StuI polymorphism and the risk for AGA, especially in white populations; however, no obvious association of the CAG or GGC triplet-repeat polymorphisms of the AR gene with risk for AGA could be confirmed. [28] Of late, the role of estrogen receptor-β polymorphisms has been identified with FPHL. [29]
Other proposed parameters implicated in the pathogenesis of FPHL include hypothyroidism and androgen-stimulating effect of prolactin. [16] The results of iron studies in FPHL have been rather conflicting [30],[31] and hence larger controlled studies and trials would be essential to derive any conclusion. Besides, as in MAGA, kenogen frequency and duration have been found to be greater in FPHL.
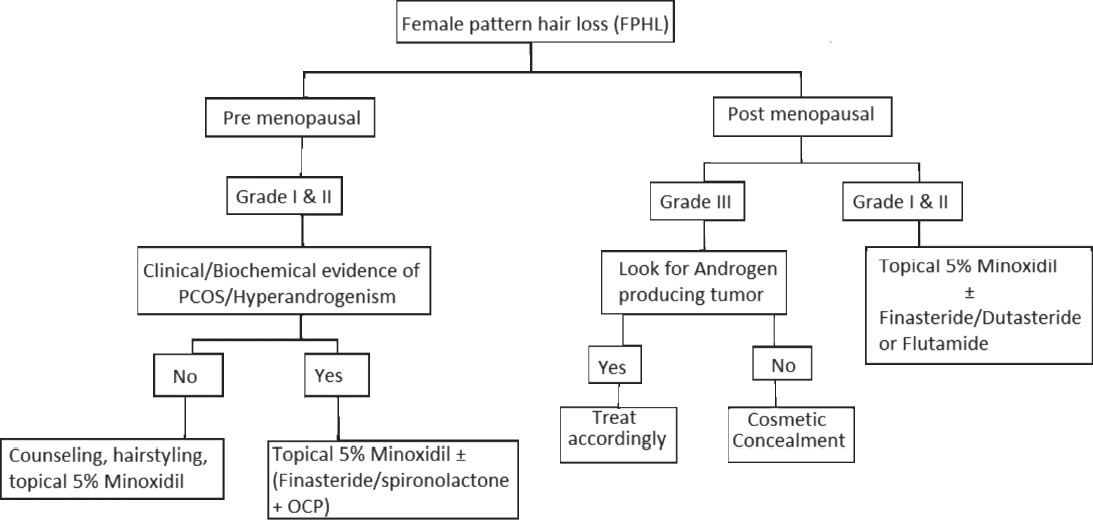
Clinical Features and Classifications
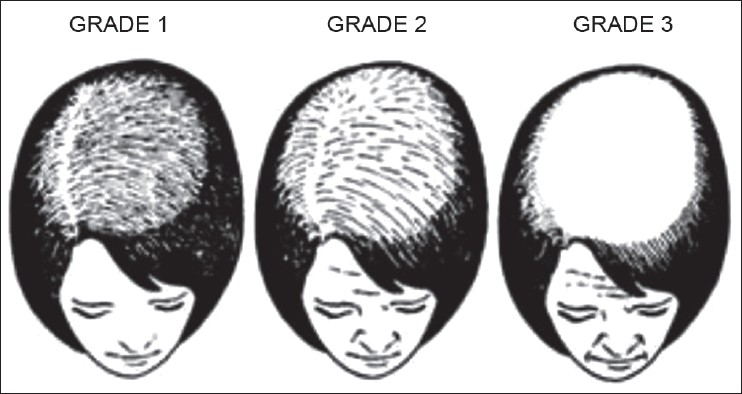
Gradual thinning of scalp hair, often over a period of several years is the usual presentation of FPHL. The hair loss may commence early in teens to as late as post-menopause. [32] A history of excessive hair shedding, unlike telogen effluvium (TE), is usually evident from the outset. In 1977, Enrick Ludwig rendered a comprehensive classification depending on the progression of the hair loss. [1] In the Ludwig′s classification, three patterns representing stages or progression of FPHL are recognized [Figure – 1].
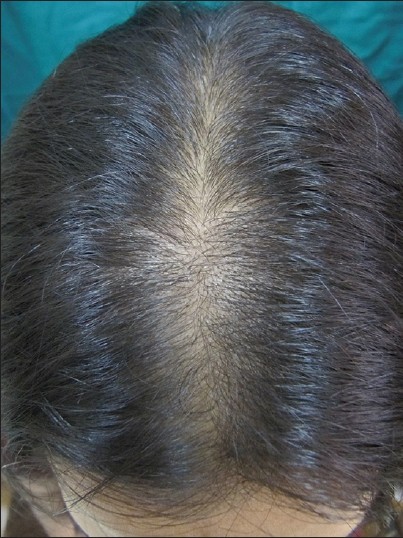
- Stage I is characterized by a perceptible thinning of hair from the anterior part of the crown with minimal widening (rarefaction) of the part width [Figure – 2]. The hair loss at this stage can easily be camouflaged. This type may manifest in young women with SAHA syndrome (seborrhea, acne, hirsutism, and alopecia), generally of ovarian origin. It may be accompanied by other manifestations of hyperandrogenism (seborrhoea, acne, hirsutism, seborrheic dermatitis, and slight menstrual alterations). Nonetheless, the biochemical levels are often normal [33]
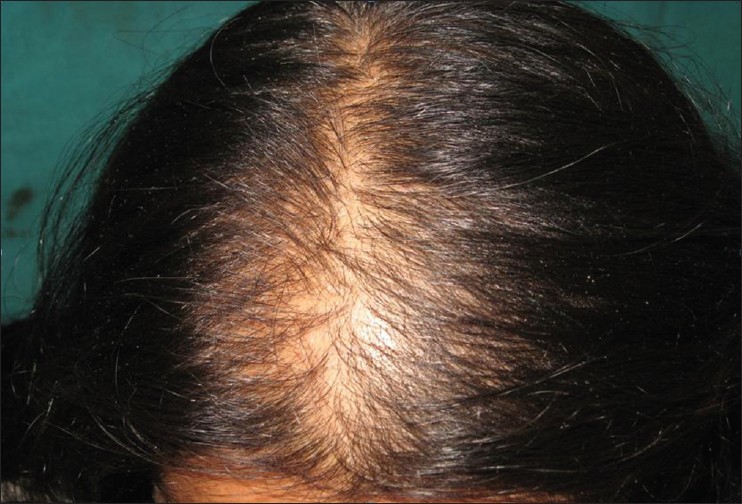
- Stage II is seen with advancing age when the rarefaction on the crown becomes more pronounced, and the number of thinner and shorter hairs increases [Figure – 3]. Camouflage of the denuded areas by special hair styles is no longer possible. An excess of androstenedione, free T, and androstanediol glucuronide may be detectable [33]
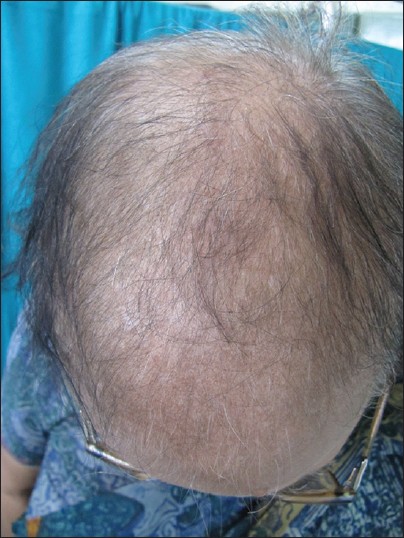
- Stage III is usually not encountered before menopause, and the crown may become literally bald [Figure – 4]. Contrary to what happens in men, a fringe of hair along the frontal hair line persists. Adrenal diseases, tumoral or not, with very high levels of androstenedione, dehydroepiandrosterone sulfates (DHEA-S), free T, sometimes of prolactin, and always of androstanediol glucuronide are the usual findings at this stage [33]
- Olsen emphasized on the frontal accentuation (or the “Christmas tree” pattern) of hair loss in FPHL. [34],[35],[36] In addition, Olsen devised a classification system, which combines the Ludwig patterns with the Savin hair density scale of eight levels (computer analysis of the temporal-parietal supra-auricular alopecia), and a classification based on three grades of overall density in either a Ludwig or a frontal accentuation pattern. [34],[36]
Women′s alopecia severity scale was an another attempt to classify five levels of FPHL by photography. Women may manifest male pattern hair loss in the form of frontotemporal recession; female androgenetic alopecia with male pattern (FAGA.M). [37] In this eventuality, Ludwig′s classification system may not be applicable. Ebling′s five-level classification [38] or Hamilton-Norwood′s classification should be used in these patients with FAGA.M.
Recently, the basic (BA) and specific (SP) classification [Figure – 5] was devised based on observed patterns of hair loss, in which the BA types represent the shape of the anterior hairline, and the SP types represent the density of hair on distinct areas (frontal and vertex). [39] There are four BA types (L, M, C, and U) and two SP types (F and V). The final type is decided by the combination of the assigned BA and SP. This classification is applicable to both FAGA.M and FPHL. In FPHL, the part width on the crown is wider than that on the occiput and may help in differentiating from chronic TE. [39]
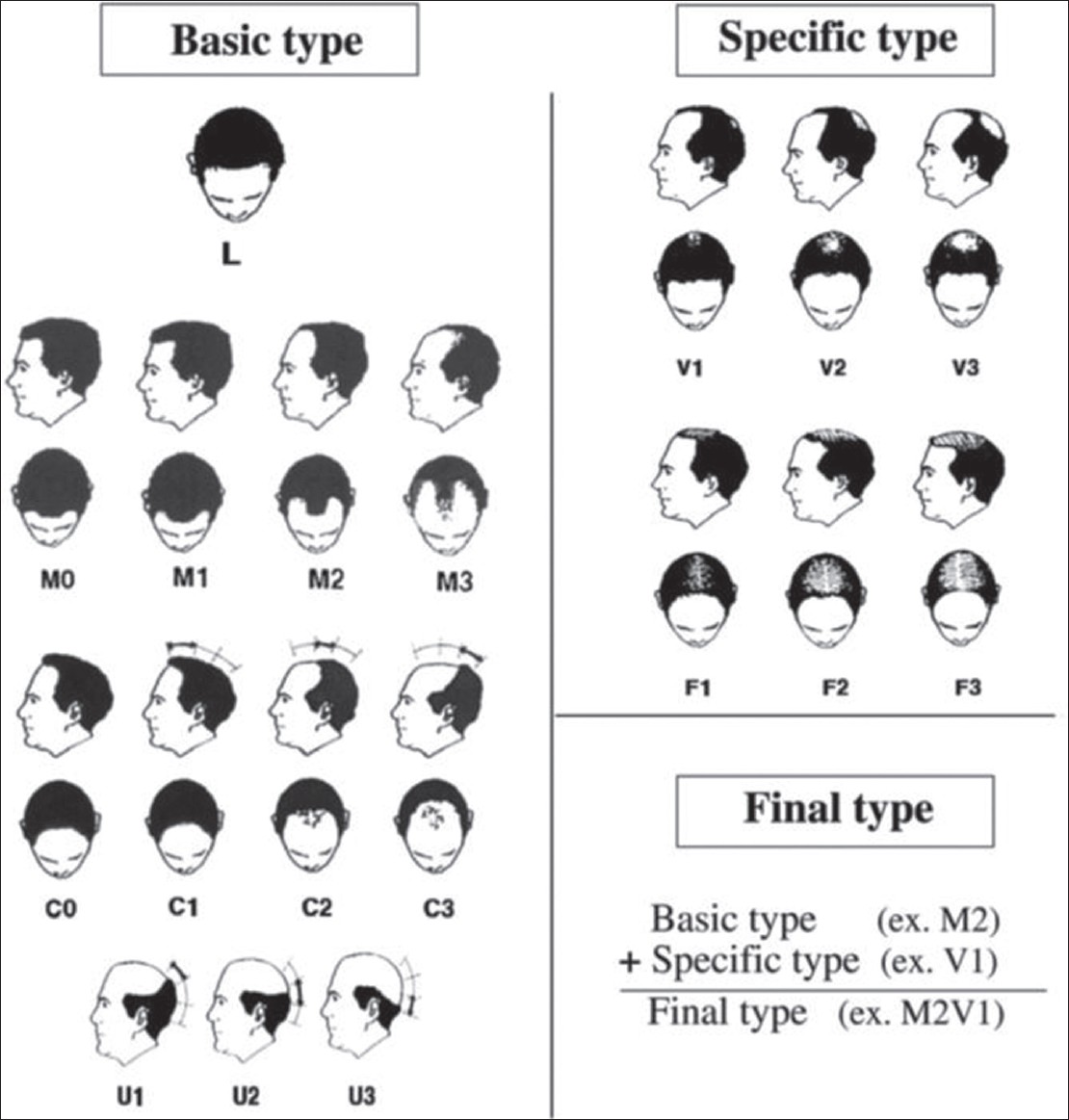 |
Female Pattern Hair Loss in Adolescents
It is worthwhile to take note of adolescent AGA, which is defined as pattern hair loss occurring in boys and girls below 18 years. [40] Adolescents with a family history of alopecia warrant careful examination and investigation. Among the ethnic groups, the prevalence of PHL among Korean adolescents has been found to be lower than Caucasians by 10-20% in the corresponding age group. [36],[40] In Korea, 3.5-6.5% of the total AGA patients were found to be adolescents. Furthermore, the proportion of female adolescents was found to be higher than the male counterparts. [32],[36],[41] In contrast, seven females of the total 43 adolescent cases had AGA in an another study from Korea. [42] Interestingly, 20% of males with AGA had a “female pattern.” In the majority of patients with AGA, the T and DHEA-S serum levels fell within the normal range. A family history of AGA in one/both parents or first-degree relatives is more common in patients who develop alopecia before adolescence than during adulthood (30.9-64.5%). [5],[41],[42],[43]
Differential Diagnoses
Differential diagnosis of FPHL encompasses the following:
- Chronic TE [Table – 1] may pose a diagnostic dilemma because some patients with FPHL may continue to shed excessive hair for months or even years before manifesting an obvious reduction in hair density. Indeed, an episode of acute TE may unmask a coexistent FPHL [44]
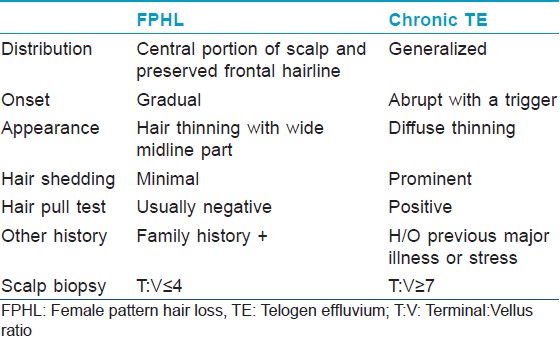 Table 1: Salient differences between female pattern hair loss and chronic telogen effluvium
Table 1: Salient differences between female pattern hair loss and chronic telogen effluvium - Diffuse alopecia areata: This disorder has an extremely acute onset with diffuse hair loss that occurs within few weeks. [45] Exclamation mark hair sign and yellow dots on dermatoscopy are helpful signs. In difficult cases, a biopsy should clarify the diagnosis
- Anagen effluvium
- Trichotillomania [Figure – 6]: Important differentiating point is the presence of hair of different length in the balding area
- Hypothyroidism: It may result in a hair loss, which may masquerade as FPHL [46]
- Systemic lupus erythematosus [32]
- Frontal fibrosing alopecia and early cicatricial alopecia.
Other conditions that need to be ruled out include nutritional deficiencies, such as iron deficiency and any chronic illnesses.
Evaluation
History
History should include information about chronic illness, nutritional deficiencies, metabolic and endocrine perturbations, and recent surgical and medical treatments. Oral contraceptives containing progesterone with a high androgenic potential or recent discontinuation of an estrogenic oral contraceptive that was taken for a long time may result in hair loss. A history regarding menstruation and the products used to care for the hair is important. [34] Women with FPHL are less likely to have a history of first-degree relatives with PHL than men with AGA. [47]
Clinical examination and hair loss evaluation methods
Besides the clinical patterns described (vide supra), certain evaluation methods aid in assessing severity of hair loss and to differentiate FPHL from other conditions. Hair evaluation methods may be non-invasive, semi-invasive, or invasive [Table – 2]. Few of these have been discussed in [Table – 3]. In addition, paired comparison of global photographs (before and after treatment) using cameras with stereotactic positioning device ensuring uniformity of view, magnification, and lighting are useful in tracking the progress of hair loss and treatment response. [48] Associated signs of hyperandrogenism such as hirsutism, moderate to severe or refractory acne, irregular menses, infertility, galactorrhea, and acanthosis nigricans, a marker for insulin resistance should be looked for.


Dermatoscopy and video dermatoscopy
The handheld dermatoscope enables appreciation of clinical features in greater detail than with the naked eye. Hairs with different diameters reflecting progressive miniaturization, brown halo at the follicular ostium, small bald areas with numerous empty follicles in post-menopausal women, and scalp pigmentation are the characteristic features of FPHL. Video dermoscopy permits more rapid and high-resolution viewing at higher magnifications than the conventional handheld dermatoscope.
TrichoScan
TrichoScan is a method of in vivo hair growth measurement that combines epiluminescence microscopy with automatic digital image analysis. [50] TrichoScan calculates a digital PTG within 15 min and is suitable for office-based hair-growth studies, being non-invasive, time-saving, and reproducible.
Histopathology
This may be required occasionally, to exclude chronic TE, diffuse alopecia areata, and cicatricial hair loss. [51],[52],[53],[54],[55] Biopsy should not be undertaken from bitemporal area as this region may have miniaturized hairs independent of AGA. A 4-mm punch biopsy deep enough to include subcutaneous fat is taken along the direction of the hair shafts. The biopsy should be sectioned horizontally as it facilitates counting of terminal, vellus and vellus-like hairs, characterization of the phase of terminal hairs cycle, and visualization of follicular streamers (vide infra). Characteristic features of FPHL are given as follows:
- Miniaturization of terminal hairs (hair shaft diameter >30 μm and thicker than the follicle′s inner root sheath) into vellus-like hairs (hair shaft diameter ≤30 μm and equal to or less than the follicle′s inner root sheath) is the cardinal feature
- Presence of follicular streamer (stelae or fibrous tract) represents the position of original terminal follicle
- Increase in the proportion of telogen hairs from a normal range of 5-10% to 15-20%. The normal ratio of 7:1 of terminal to vellus decreases to 1.9:1 (both sexes combined) or 2.2:1 in FPHL
- A perifollicular infiltrate, predominantly lymphohistiocytic, may be present around the upper or lower follicle
- Perifollicular fibrosis, a poor prognostic indicator, may be present in PHL.
Biochemical
Most women with FPHL do not have an underlying detectable hormonal abnormality. Nevertheless, women with signs of hirsutism, moderate to severe and/or treatment-refractory adult acne, acanthosis nigricans, irregular menses, and or galactorrhea should be screened for androgen excess.
- Free and/or total T and DHEA-S are indispensable biochemical parameters
- Serum prolactin levels.
If T is greater than 2.5 times of normal or >200 ng/dL, or DHEA-S is greater than 2 times or >700 μg/dL in pre-menopausal or >400 μg/dL in post-menopausal women, imaging should be undertaken for possible underlying neoplasm. [56] Female patients with hyperprolactinemia may experience hirsutism, along with a FPHL phenotype. [57] Serum 17-OH progesterone levels, especially before and after ACTH (Adrenocorticotropic hormone) stimulation test, during the follicular phase of the menstrual cycle are an appropriate screening test for congenital adrenal hyperplasia due to 21-hydroxylase deficiency. If raised, appropriate imaging studies may be required to scan the adrenals. Prostate-specific antigen, a marker of hyperandrogenism, may also be evaluated. [58] Cushing′s disease may be assessed by measuring 24h urine cortisol levels and dexamethasone suppression test. Free androgen index test [FAI = total T (nmol/L) × 100/SHBG (nmol/L)] of more than 5 is indicative of hyperandrogenemia. [59],[60]
Treatment
Counselling
Women with hair loss are more likely to have a lowered self-esteem and lowered quality of life than men. Thus, the patient should be adequately counseled about the need for detailed evaluation, availability of effective medical treatments, realistic expectations, and the need for long term, indefinite treatment to maintain response. Psychotherapy and anxiolytic therapy may also be considered in extreme cases as it is possible that patients who are depressed perceive even normal hair shedding in an exaggerated manner. [61] They should also be advised to refrain from any potentially traumatic hair cosmetic procedures.
Nutritional supplementation
The benefit of oral supplementation with amino-acids, biotin, zinc, and other micronutrients in hair loss of any origin is controversial. While in one study, 6-month supplementation with L-cystine, medicinal yeast and B-complex vitamins in otherwise healthy women (25-65 years) with TE showed normalization of the anagen hair rate by TrichoScan analysis, [62] the relevance of such supplementation in FPHL is controversial and needs further research. Similarly, an apparent lack of correlation between low serum ferritin levels (<10 g/l) in apparently healthy women with FPHL, CTE, or both raised doubt if iron supplementation will be helpful. [63]
Medical treatment
Pharmacological treatments for FPHL may be topical and oral. They can also be divided into drugs with androgen-dependent and independent mechanisms of action.
Topical
Minoxidil topical solution (MNX): MNX first approved for use in MAGA is now licensed for use in FPHL in a 2% formulation as twice daily application of 1 ml solution over dry scalp. Minoxidil increases follicular vascularity (as a potassium channel opener), prolongs anagen, shortens telogen, and converts partially miniaturized (intermediate) to terminal hairs.
Trials of 2% MNX in FPHL using hair counts as a primary endpoint have shown a mean increase in hair growth of 15-33% in the treated versus vehicle-control groups. [64],[65] A multicenter 32-week trial revealed that the number of non-vellus hairs in a 1-cm 2 evaluation site was increased by an average of 23 hairs in the 2% minoxidil group versus 11 hairs in placebo. The overall growth was moderate in 13% of treated versus 6% in placebo, and minimal growth in 50% versus 33% in placebo group. [66] No serious medical adverse effects were reported.
In a 48-week study of 381 women with FPHL, both 2% and 5% MNX applied twice daily were found to be superior than placebo, and 5% MNX demonstrated statistical superiority over the 2% in terms of the patient assessment of treatment benefit, but not in clinical efficacy. Facial hypertrichosis, a known adverse effect of MNX was more in 5% group. [67] In an another 24-week study comprising 113 women with AGA, 5% minoxidil foam applied once daily was found to be as slightly more effective than 2% MNX applied twice daily, and was preferred by patients due to less pruritus and ease of single daily application. [68] Combination of MNX with aminexil (1.5%), a reverser of perifollicular fibrosis, with tretinoin and azelaic acid, and with topical finasteride are also commercially available, but their superiority over MNX alone in FPHL is not established.
Similar to MAGA, it is likely that the effect will reverse after cessation of treatment. Patient should be counseled about the increased hair loss following MNX application followed by stabilization and then noticeable increase in 8-12 weeks that usually peaks after 16 weeks. Minoxidil is a safe treatment; most common adverse effects being unsightly deposits on the hair-simulating dandruff, scalp irritation, and occasionally contact dermatitis that is due to vehicle propylene glycol rather than active molecule. Availability of MNX in gel and foam formulations enhances treatment acceptability. Secondly, as compared with men, women are more likely to develop hypertrichosis on the face (typically cheeks and forehead) and even on other remote sites especially with higher concentrations (5% > 2%). It is reversible on treatment break or reduction of concentration (within 4-6 months) and in some without cessation of treatment. [69] This side-effect may be minimized by wiping face and ears with a wet cloth after MNX application. The MNX being reasonably safe and effective should be considered as the first-line treatment for FPHL.
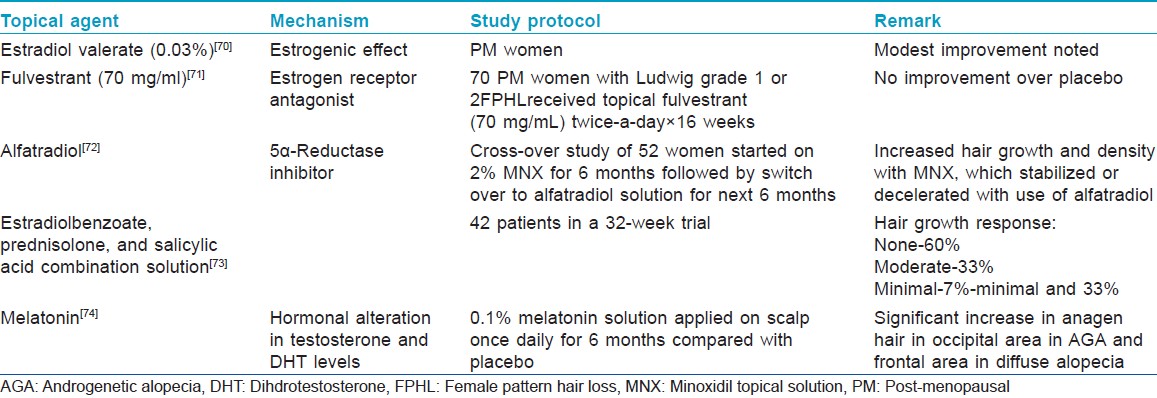
Oral
Androgen-dependent oral drugs for FPHL include 5α-reductase inhibitors and anti-androgens. Due to their propensity to cause feminization of the male fetus, they are contra-indicated in pregnant women and it is advisable to combine them with oral contraceptive pills (OCPs), especially in pre-menopausal women.
5α-Reductase inhibitors: Although compared with men, women have lower levels of this enzyme in the dermal papilla; inhibitors of this enzyme have been found to be effective in arresting hair loss in FPHL.
Finasteride
Finasteride, an inhibitor of 5α-reductase type II enzyme, suppresses systemic as well as hair follicular androgen activity by inhibiting conversion of T to its more active form, that is, DHT. The efficacy of this drug in FPHL has been reported to be variable in different studies. In an earlier double-blind, placebo-controlled, randomized study, finasteride 1 mg/day given over 1 year to 137 post-menopausal women failed to show any benefit. [75] However, smaller case reports and case series that employed relatively higher doses (1.25 mg/day in four cases with hyperandrogenism, 2.5-5 mg/day in five normoandrogenic cases, and 5 mg/week in one normoandrogenic post-menopausal woman) found it to be effective. [76],[77],[78] In the study by Iorizzo et al., 62% pre-menopausal normoandrogenic women with FPHL showed improvement with global photography following 2.5 mg/day of finasteride use for 12 months. [79] However, they were also taking OCPs and thus the benefit may have been due to combination therapy. However, in a recent larger open-label study by Yeon et al. [80] of 87 normoandrogenic pre- and post-menopausal women, significant improvement in mean hair density and hair thickness was observed in upto 80% patients after 12 months of 5 mg/day finasteride therapy as evident from PTG and global photography. Interestingly, there was no significant difference in the clinical improvement between the pre-and post-menopausal groups. Adverse effects such as headache, menstrual irregularity, dizziness, and increased body hair growth were noted in four patients, and were mild and temporary. As the pre-menopausal women in this study were using non-hormonal contraceptive measures, isolated effect of finasteride seems logical. It is thus likely that for FPHL, higher dosages of finasteride (2.5-5 mg/day) should be given in contrast to 1 mg/day used for MAGA. However, large-scale studies, preferably double-blinded or placebo-controlled are needed to reaffirm the efficacy and optimal dosage of finasteride for FPHL.
Finasteride is generally well-tolerated. Infrequent side-effects in female patients include breast tenderness and increased libido, which are most common in the first year of therapy and tend to diminish with continued use. Whenever used in pre-menopausal women, it should always be combined with OCPs or other effective method of contraception to prevent inadvertent conception and feminization of male fetus.
Dutasteride inhibits both type I and type II 5α-reductase enzyme and is three times more potent blocker of type II enzyme than finasteride. It has been used successfully in the treatment of male AGA. After the first reported benefit of 6 months course of this drug in a 46-year-old woman with refractory FPHL, it was tried in 23 normoandrogenic post-menopausal women with AGA, of which 12 showed a clinically evident improvement, more in the occiput. [33],[81] It has also been shown to be beneficial when used as mesotherapy injections containing dutasteride 0.5 mg, biotin 20 mg, pyridoxin 200 mg, and D-panthenol 500 mg given over 12 sessions. Of 86 FPHL patients treated, 62.8% responded results substantiated by photographic assessment, hair diameter, and patient self-assessment. [82] The optimal dose of dutasteride for FPHL is not established, although 2.5 mg/day (same as in men) seems to be practical. It should be noted that blood levels of dutasteride persist for a longer time than finasteride, an important consideration if used in women of reproductive age group.
Anti-androgens
Anti-androgens act primarily through blockade of the ARs.
Cyproterone acetate: This drug blocks ARs and inhibits gonadotropin- releasing hormone. In many countries such as India, it is available in combination with estradiol as an OCP. The beneficial role of cyproterone acetate (CPA) in isolation or in combination with OCPs in reducing hair shedding and increasing hair density is known and seems to be greater in patients with evidence of hyperandrogenism. In two studies using Diane-35 for 6-9 months and as 2 mg CPA plus 50 μg ethinylestradiol daily plus additional 20 mg CPA on days 5-20 of the menstrual cycle, respectively, decrease in shedding and thinning of hair were observed. [83],[84] In yet another study, pre-menopausal women received CPA 100 mg daily for the first 10 days of each menstrual cycle for 12 months in combination with an oral contraceptive, while post-menopausal women received 50 mg daily of CPA continuously. At the end of 12 months, 35 (44%) women had clearly visible hair regrowth, 35 (44%) had no change in hair density, and 10 (12%) had reduced hair density. [85] While one randomized comparative trial revealed that minoxidil 2% was more effective in women without evidence of hyperandrogenism, whereas CPA was more effective in those with hyperandrogenism, the efficacy of CPA versus spironolactone was found comparable in another trial. [86],[87] The overall balance of evidence supports a role for CPA in FPHL, especially in presence of hyperandrogenism.
The most effective treatment dose seems to be 100 mg/day on days 5-15 of the menstrual cycle supplemented by 50 μg ethinylestradiol on days 5-25. [88] The adverse effects include menstrual disturbances, weight gain, loss of libido, depression, breast tenderness, and gastrointestinal upsets.
Spironolactone, an anti-androgen, is often used for treating hirsutism, acne, and seborrhea. Recently, efforts have been made to explore its role in FPHL. Eighty women with biopsy-proven FPHL were studied, most without biochemical hyperandrogenism; 40 received spironolactone 200 mg daily, and 40 received CPA. Both drugs were found to be effective. [85] A combination of spironolactone and 5% minoxidil has also been found to be efficacious. [89] It was found to be effective at a daily dose of 100-200 mg over a period of 6 months in seven patients of FPHL with a mean age of 37 years. [90] The response, however, was assessed subjectively.
Drospirenone, a 17α-spironolactone derivative possesses progestagenic, anti-androgenetic, and anti- aldosteronic activities. It is administered in a dose of 3 mg/day in combination with 30 μg of ethinylestradiol during a 21-day cycle. It has been recommended for the treatment of SAHA and is devoid of fluid retention property. [33]
Flutamide, a selective anti-androgen with a non-steroidal structure, has been evaluated in FPHL and found to be effective when given for at least a year in dose of 250 mg/day. [91] In an another study, a cohort of 101 women with FPHL had received, yearly reducing doses (250, 125, and 62.5 mg/day) of flutamide for 4 years; 33 patients were treated with flutamide alone and 68 received additional oral contraceptive. Both groups showed a marked decrease in alopecia scores after 12 months with maximum response at 2 years that was maintained subsequently. Four percent of patients dropped due to hepatic dysfunction during the initial year. [92] Two randomized studies found that flutamide resulted in greater improvements when compared with spironolactone, finasteride, and CPA as well. [85],[93] However, adverse effects caused by flutamide are potentially severe and include dose-related hepatic dysfunction (3/10,000 users) and breast tenderness. Thus, serial liver transaminases should be monitored in the first few months of treatment, and drug should be discontinued if transaminase levels exceed twice the normal limits. [94]
Surgical treatment
Hair transplantation is advantageous in being less invasive and a permanent hair cover can be re-established. A number of procedures have been tried, of these, follicular unit transplantation (FUT) is in vogue. In FUT, follicular units of 1-4 hairs are transplanted in high densities. Hair transplantation works on the principle of “donor dominance,” which means that the hairs from the donor region (the occiput) inherently lack the androgen-sensitive receptors, a property that is retained even after transplantation into the fronto-parietal region. It is indicated in patients with extensive hair loss or thinning of frontal scalp, but with the pre-requisite of high-donor hair density. [95]
Newer therapies
Pulsed electromagnetic treatment has been used to treat FPHL occasionally, although cost and availability limit its use. [96] Twenty-seven patients with FPHL completed a 5-month schedule of 1550-nm fractional erbium-glass laser treatment resulting in significant increase in both mean hair thickness and density in 24 patients as evaluated by PTG and global photographs. [97]
Platelet-rich plasma therapy, comprising injection of patient′s own plasma enriched with platelet levels of ≥10,00,000/μL, has recently shown modest benefits in 26 individuals (16 men, and 10 women) with thin hair in fronto-parietal scalp. [98] However, long-term efficacy and protocols for repeat sessions for FPHL are still not clear.
Cosmetic concealment
Camouflaging products and other concealment techniques can effectively mask areas of visible hair loss summary of which is given below: [94]
- Mild-moderate loss: Hair fibers (keratin-made), masking lotions, topical shading, and scalp spray thickeners may be used. These are compatible with topical minoxidil and are easily removed with shampooing
- Moderate-severe loss: Integration hairpiece (fabric- or skin-like material) or wig may used
- Localized or diffuse patches: Hats, scarves, bandanas, and turbans may be used.
 |
Undefined modalities
There are several molecules being propagated in the market in various forms namely amino-acids, iron supplements in absence of iron deficiency, vitamins (biotin and niacin derivates), proanthocyanidine, millet seed (silicic acid, aminoacids, vitamins, and minerals), marine extract and silica component, Chinese herbals, ginkgo biloboa, aloe vera, ginseng, bergamot, hibiscus, sorphora, caffeine, retinoids, cyclosporine, electromagnetic/-static field, low-level laser, aminexil, glyceroloxyesters, silicium, minerals, niacin derivates, mesotherapy, saw palmetto, β-sitosterol, polysorbate 60, green tea, cimicifuga racemosa, ketoconazol, zinc pyrithione, and botulinum toxin. These suffer from sufficient scientific evidence to be prescribed in AGA. [99]
Female AGA, Metabolic Syndrome, and Cardiovascular Risk
In contrast to documented association of AGA and coronary artery disease (CAD) in men, few studies have focused on this association in women. In a cross-sectional study performed in 66 non-obese women (24-44 years old), compared with 25 healthy controls, women with AGA (41 patients) had impaired glucose tolerance, higher fasting glucose, c-peptide and insulin levels, and homeostatic model assessment-insulin resistance (HOMA-IR) suggestive of insulin resistance. [100] In a case-control study comprising 154 participants (77 patients with early-onset AGA (40 male and 37 female) and same number of age- and sex-matched healthy controls), comparison of patients and controls revealed the following: Metabolic syndrome in 60% versus 12.5% in men and 48.6% versus 8.1% in women; atheromatous plaques in 32.5% versus 7.5% in men and 27% versus 8.1% in women; in addition, levels of aldosterone, insulin, fibrinogen, C-reactive protein, and D-dimers were significantly higher in female patients with AGA versus their respective controls. [101] Based on the results of these studies, even women with early-onset AGA should be screened to detect risk of CAD. Oral glucose tolerance test, HOMA-IR, and carotid artery Doppler examination seem to be useful screening modalities for this purpose.
[“source=ijdvl”]
